Density definition, the state or quality of being dense; compactness; closely set or crowded condition. What is Density? Density Formula Density = Mass divided by volume. The units used vary depending on the units of the mass and volume used for the calculation. If the mass was measured in kg and volume in cm³ the units for density would be in kg/cm³. Something else that can be confusing is mass.
- Definition of density 1: the quality or state of being dense measures of traffic density the density of the cake felt that the candidate's density on the subject of equality was alarming the density of her prose.
- Density is defined as the ratio of the mass of an object to the volume of space the object takes up. Mathematically, we would say D = M/V. Density will determine if you sink or float when put into a liquid, like water. Take 8 same-size plastic Easter eggs that come.
- Optical density is the process of transmission of light or other electromagnetic radiation by matter. The process of emission and absorption depends on the wavelength of the radiations, which includes the interaction between fundamental particles like electrons, atoms, ions, etc.
By the end of this lesson, you will be able to:
- calculate a single variable (density, mass or volume) from the density equation
- calculate specific gravity of an object, and
- determine whether an object will float or sink given its density and the density of its surroundings.

An introduction to density
Density is the mass of an object divided by its volume.Density often has units of grams per cubic centimeter (g/cm3). Remember, grams is a mass and cubic centimeters is a volume (the same volume as 1 milliliter).
Density is a fundamental concept in the sciences; you will see it throughout your studies. It is used quite often in identifying rocks and minerals since the density of substances rarely changes significantly. For example, gold will always have a density of 19.3 g/cm3; if a mineral has a density other than that, it isn't gold.
You probably have an intuitive feeling for density in the materials you use often. For example, sponges are low in density; they have a low mass per unit volume. You are not surprised when a large sponge is easy to lift. In contrast, iron is dense. If you pick up an iron skillet, you expect it to be heavy.
Students, and even teachers, often confuse mass and density. The words heavy and light on their own refer to mass, and not density. A very large sponge may weigh a lot (have a high mass), but its density is low because it still weighs very little per unit of volume. For density, you also need to consider the size, or volume, of the object.
How do I determine density?
Another tricky thing about density is that you can't add densities. If I have a rock that is made up of two minerals, one with a density of 2.8 g/cm3, and one with a density of 3.5 g/cm3, the rock will have a density between 3.5 and 2.8 g/cm3, not a density of 6.3 g/cm3. This is because both the mass and the volume of the two minerals will be added, and so when they are divided to get the density the result will be between the two.
Typical densities for gasses are on the order of thousandths of grams per cubic centimeter. Liquids often have densities of about 1.0 g/cm3, and indeed, fresh water has a density of 1.0 g/cm3. Rocks often have a density around 3 g/cm3, and metals often have densities above 6 or 7 g/cm3.
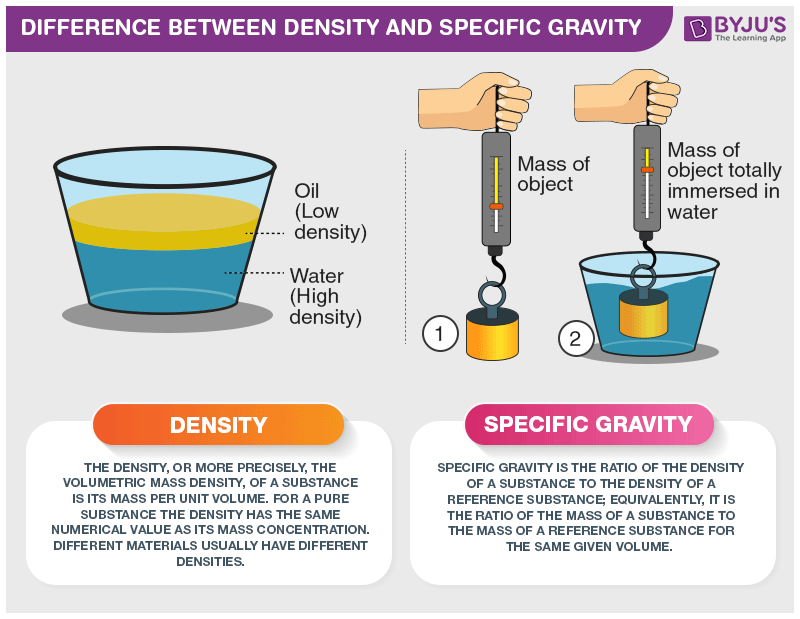
How do I calculate specific gravity?
To calculate the specific gravity (SG) of an object, you compare the object's density to the density of water:
Because the density of water in g/cm3 is 1.0, the SG of an object is will be almost the same as its density in g/cm3. However, specific gravity is a unitless number, and is the same in the metric system or any other measurement system. It is very useful when comparing the density of two objects. Since specific gravity is unitless, it doesn't matter whether the density was measured in g/cm3 or in some other units (like lbs/ft3).
You have a sample of basalt with density 210 lbs/ft3. The density of water is 62.4 lbs/ft3. What is the specific gravity of the basalt?So we divide the basalt (210 lbs/ft3) by the density of water (62.4 lbs/ft3), and get S.G.= 3.37.
Why should I calculate density or specific gravity?
Densities are critical for many uses. One of the most critical is that the density of a substance will determine if it will float on another. Less dense substances will float on (or rise through) more dense substances. Here are some examples of how this explains everyday occurrences:
- Have you wondered why hot air balloons rise? When the air is heated, it becomes less dense until the balloon's total density is less than that of the atmosphere; A hot air balloon is literally floating on the denser, colder air.
- Have you ever noticed in a lake or the ocean that water is warmer at the surface and colder at the bottom? This is because the warmer water is slightly less dense and, as a result floats on the denser, colder water
- Do you know why volcanoes erupt? This huge boat weighs a lot, but it its density must be less than 1.0 g/cm3 because it is floating.The main reason that magma rises to the surface to erupt at volcanoes is because it is less dense than the rocks that surround it.
Where is density used in the geosciences?
- Isostasy - determining how high continents will sit on the mantle
- Plate tectonics - mechanisms that drive plate tectonics
- Minerals - determining the name of a mineral through its density
- Rocks - determining the name and composition of a rock by its density
- The hypsometric curve - examining the causes of elevation variation on Earth
- Oceanography - some ocean currents and ocean circulation is controlled by density
Next Steps
Densities Def
I am ready to PRACTICE!
If you think you have a handle on all of the things listed above click on this bar to try some practice problems with worked answers!Or, if you want even more practice, see the links below
More help with Density
Edinformatics on-line lab on mass, volume and density is put together by NYU. It allows you to look at pictures of measurements and to enter data.Density Dependent Factors
Hyperphysics, at the Georgia State has a page about density and a density converter. This includes several related pages including instructions of measuring density using the Archimedes principle.
Wikipedia's specific gravity page has an explanation of what specific gravity is and how it is used and even discusses its use in the geosciences and mineralogy. However, the content of Wikipedia articles may change and so you may want to be cautious.
Wikipedia's Density page has a general discussion of density and its history, calculation, and units. However, the content of Wikipedia articles may change and so you may want to be cautious.
Density Of Steel
This page was written and compiled by Dr. Eric M. Baer, Geology Program, Highline Community College, and Dr. Jennifer M. Wenner, Geology Department, University of Wisconsin OshkoshComplete list of density units for conversion
Density Of Air
- kilogram/cubic meter
- 1 gram/cubic centimeter = 1000 kilogram/cubic metergram/cubic centimeter to kilogram/cubic meter, kilogram/cubic meter to gram/cubic centimeter
- 1 kilogram/cubic centimeter = 1000000 kilogram/cubic meterkilogram/cubic centimeter to kilogram/cubic meter, kilogram/cubic meter to kilogram/cubic centimeter
- 1 gram/cubic meter [g/m^3] = 0.001 kilogram/cubic metergram/cubic meter to kilogram/cubic meter, kilogram/cubic meter to gram/cubic meter
- 1 gram/cubic millimeter = 1000000 kilogram/cubic metergram/cubic millimeter to kilogram/cubic meter, kilogram/cubic meter to gram/cubic millimeter
- 1 milligram/cubic meter = 1.0E-6 kilogram/cubic metermilligram/cubic meter to kilogram/cubic meter, kilogram/cubic meter to milligram/cubic meter
- 1 milligram/cubic centimeter = 1 kilogram/cubic metermilligram/cubic centimeter to kilogram/cubic meter, kilogram/cubic meter to milligram/cubic centimeter
- 1 milligram/cubic millimeter = 1000 kilogram/cubic metermilligram/cubic millimeter to kilogram/cubic meter, kilogram/cubic meter to milligram/cubic millimeter
- 1 exagram/liter [Eg/L] = 1.0E+18 kilogram/cubic meterexagram/liter to kilogram/cubic meter, kilogram/cubic meter to exagram/liter
- 1 petagram/liter [Pg/L] = 1.0E+15 kilogram/cubic meterpetagram/liter to kilogram/cubic meter, kilogram/cubic meter to petagram/liter
- 1 teragram/liter [Tg/L] = 1000000000000 kilogram/cubic meterteragram/liter to kilogram/cubic meter, kilogram/cubic meter to teragram/liter
- 1 gigagram/liter [Gg/L] = 1000000000 kilogram/cubic metergigagram/liter to kilogram/cubic meter, kilogram/cubic meter to gigagram/liter
- 1 megagram/liter [Mg/L] = 1000000 kilogram/cubic metermegagram/liter to kilogram/cubic meter, kilogram/cubic meter to megagram/liter
- 1 kilogram/liter [kg/L] = 1000 kilogram/cubic meterkilogram/liter to kilogram/cubic meter, kilogram/cubic meter to kilogram/liter
- 1 hectogram/liter [hg/L] = 100 kilogram/cubic meterhectogram/liter to kilogram/cubic meter, kilogram/cubic meter to hectogram/liter
- 1 dekagram/liter [dag/L] = 10 kilogram/cubic meterdekagram/liter to kilogram/cubic meter, kilogram/cubic meter to dekagram/liter
- 1 gram/liter [g/L] = 1 kilogram/cubic metergram/liter to kilogram/cubic meter, kilogram/cubic meter to gram/liter
- 1 decigram/liter [dg/L] = 0.1 kilogram/cubic meterdecigram/liter to kilogram/cubic meter, kilogram/cubic meter to decigram/liter
- 1 centigram/liter [cg/L] = 0.01 kilogram/cubic metercentigram/liter to kilogram/cubic meter, kilogram/cubic meter to centigram/liter
- 1 milligram/liter [mg/L] = 0.001 kilogram/cubic metermilligram/liter to kilogram/cubic meter, kilogram/cubic meter to milligram/liter
- 1 microgram/liter [µg/L] = 1.0E-6 kilogram/cubic metermicrogram/liter to kilogram/cubic meter, kilogram/cubic meter to microgram/liter
- 1 nanogram/liter [ng/L] = 1.0E-9 kilogram/cubic meternanogram/liter to kilogram/cubic meter, kilogram/cubic meter to nanogram/liter
- 1 picogram/liter [pg/L] = 1.0E-12 kilogram/cubic meterpicogram/liter to kilogram/cubic meter, kilogram/cubic meter to picogram/liter
- 1 femtogram/liter [fg/L] = 1.0E-15 kilogram/cubic meterfemtogram/liter to kilogram/cubic meter, kilogram/cubic meter to femtogram/liter
- 1 attogram/liter [ag/L] = 1.0E-18 kilogram/cubic meterattogram/liter to kilogram/cubic meter, kilogram/cubic meter to attogram/liter
- 1 pound/cubic inch [lb/in^3] = 27679.904710191 kilogram/cubic meterpound/cubic inch to kilogram/cubic meter, kilogram/cubic meter to pound/cubic inch
- 1 pound/cubic foot [lb/ft^3] = 16.018463374 kilogram/cubic meterpound/cubic foot to kilogram/cubic meter, kilogram/cubic meter to pound/cubic foot
- 1 pound/cubic yard [lb/yd^3] = 0.5932764213 kilogram/cubic meterpound/cubic yard to kilogram/cubic meter, kilogram/cubic meter to pound/cubic yard
- 1 pound/gallon (US) = 119.8264273167 kilogram/cubic meterpound/gallon (US) to kilogram/cubic meter, kilogram/cubic meter to pound/gallon (US)
- 1 pound/gallon (UK) = 99.7763726631 kilogram/cubic meterpound/gallon (UK) to kilogram/cubic meter, kilogram/cubic meter to pound/gallon (UK)
- 1 ounce/cubic inch [oz/in^3] = 1729.9940443869 kilogram/cubic meterounce/cubic inch to kilogram/cubic meter, kilogram/cubic meter to ounce/cubic inch
- 1 ounce/cubic foot [oz/ft^3] = 1.0011539609 kilogram/cubic meterounce/cubic foot to kilogram/cubic meter, kilogram/cubic meter to ounce/cubic foot
- 1 ounce/gallon (US) = 7.4891517073 kilogram/cubic meterounce/gallon (US) to kilogram/cubic meter, kilogram/cubic meter to ounce/gallon (US)
- 1 ounce/gallon (UK) = 6.2360232914 kilogram/cubic meterounce/gallon (UK) to kilogram/cubic meter, kilogram/cubic meter to ounce/gallon (UK)
- 1 grain/gallon (US) = 0.017118061 kilogram/cubic metergrain/gallon (US) to kilogram/cubic meter, kilogram/cubic meter to grain/gallon (US)
- 1 grain/gallon (UK) = 0.0142537675 kilogram/cubic metergrain/gallon (UK) to kilogram/cubic meter, kilogram/cubic meter to grain/gallon (UK)
- 1 grain/cubic foot [gr/ft^3] = 0.0022883519 kilogram/cubic metergrain/cubic foot to kilogram/cubic meter, kilogram/cubic meter to grain/cubic foot
- 1 ton (short)/cubic yard = 1186.552842515 kilogram/cubic meterton (short)/cubic yard to kilogram/cubic meter, kilogram/cubic meter to ton (short)/cubic yard
- 1 ton (long)/cubic yard = 1328.9391836174 kilogram/cubic meterton (long)/cubic yard to kilogram/cubic meter, kilogram/cubic meter to ton (long)/cubic yard
- 1 slug/cubic foot [slug/ft^3] = 515.3788183932 kilogram/cubic meterslug/cubic foot to kilogram/cubic meter, kilogram/cubic meter to slug/cubic foot
- 1 psi/1000 feet = 2.3066587258 kilogram/cubic meterpsi/1000 feet to kilogram/cubic meter, kilogram/cubic meter to psi/1000 feet
- 1 Earth's density (mean) = 5517.9999999999 kilogram/cubic meterEarth's density (mean) to kilogram/cubic meter, kilogram/cubic meter to Earth's density (mean)
